A Gaseous Mixture of O2 and N2
At NTP pressure is always equal to 1 a t m and temperature is equal to 27315 K. Calculate Partial pressure of O2.
Solved A Gaseous Mixture Of O2 And N2 Contains 35 8 Chegg Com
A 2237 B 05.

. What is the partial pressure of oxygen in the mixture if the total pressure is 625 mmHg. NOW CONSIDER THE VOLUME OF N2 BE a L. You must use the data you have been given.
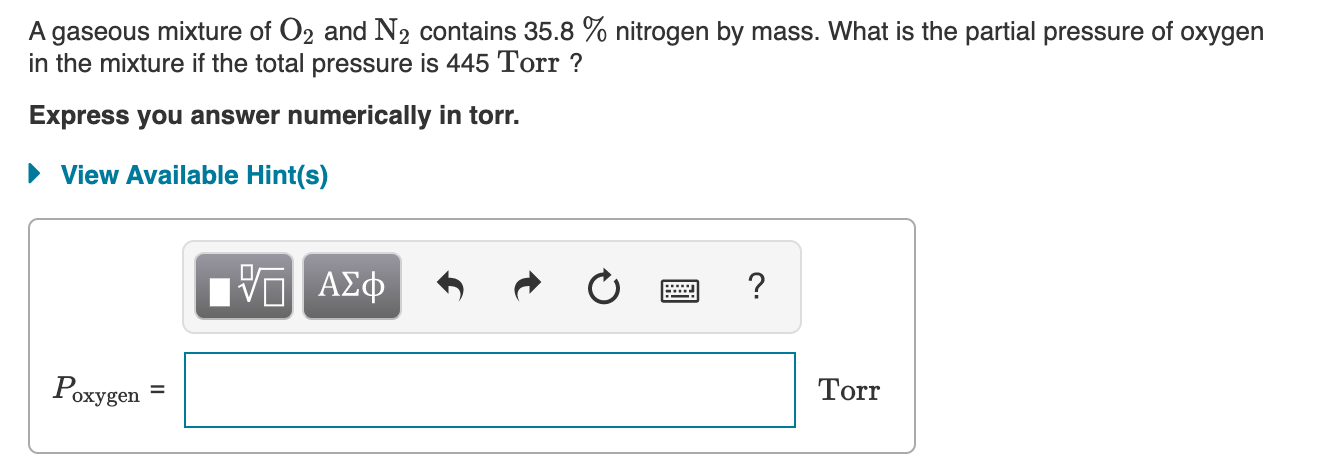
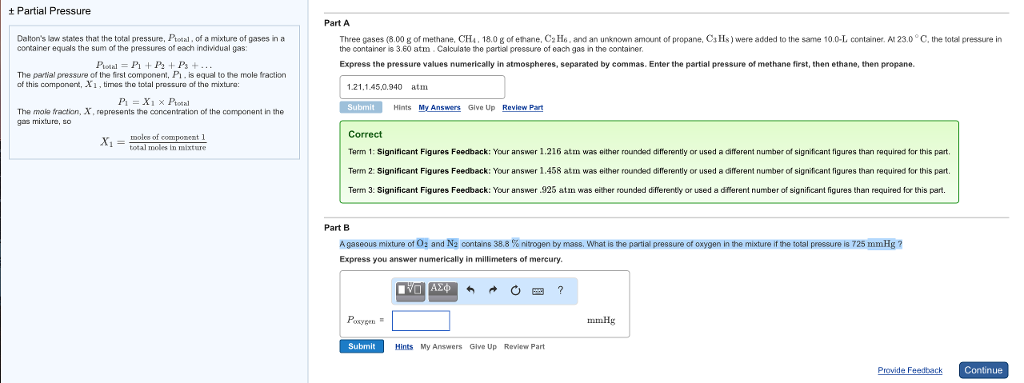
Ideal gas equation in terms of density. The ratio of active masses of 22g of CO2 3g of H2 and 7g of N2 in a gaseous mixture. Up to 256 cash back A gaseous mixture of O2 and N2 contains 398 of nitrogen by mass.
20 ml of a gaseous mixture of N2 and H2 gases is mixed with 8 ml O2 and the mixture is firedif the final volume becomes 13 ml the volume percent of N2 in the. What is the partial pressure of oxygen in the mixture if the total pressure is 685 mmHg. 130 mmHg CO2 210 mmHg Ar and 182 mmHg O2.
D is the density of gas mixture whose given value is 13 g. Express you answer numerically in millimeters of mercury. Cl2O gas decomposes as.
312242 Gaseous mixture 20 ppm H2S 60 ppm CO 145 CH4 15 O2 N2 MSA mix 4 5 weeks 23867 312117 Gaseous mixture 25 ppm H2S 100 ppm CO 22 CH4 15 O2 N2 BW mix Available 23867. A gaseous mixture of N2 O2 containing 60 by mole N2 is allowed to react with each other according to the following equaion N2. 14 19 Partial pressure of Oxygen Mole fraction of Oxygen Total pressure.
A gaseous mixture of O2 and N2 contains 328 nitrogen by mass How do i figure out wat the mass is. P M d R T. 7 C 131 D 13.
The mole fraction of oxygen is_____. The density of the mixture of O2 and N2 at STP is 13 gl. Whah is the partial pressure of oxygen in the mixture if the totally pressure is 766 mmHg.
C1207g Cl2g O2g A partially decomposed gaseous mixture is allowed to effuse through a pin-hole and the gas coming out initially was analysed. Express you answer numerically in millimeters of mercury. A gaseous mixture of O2 and N2 contains 358 nitrogen by mass.
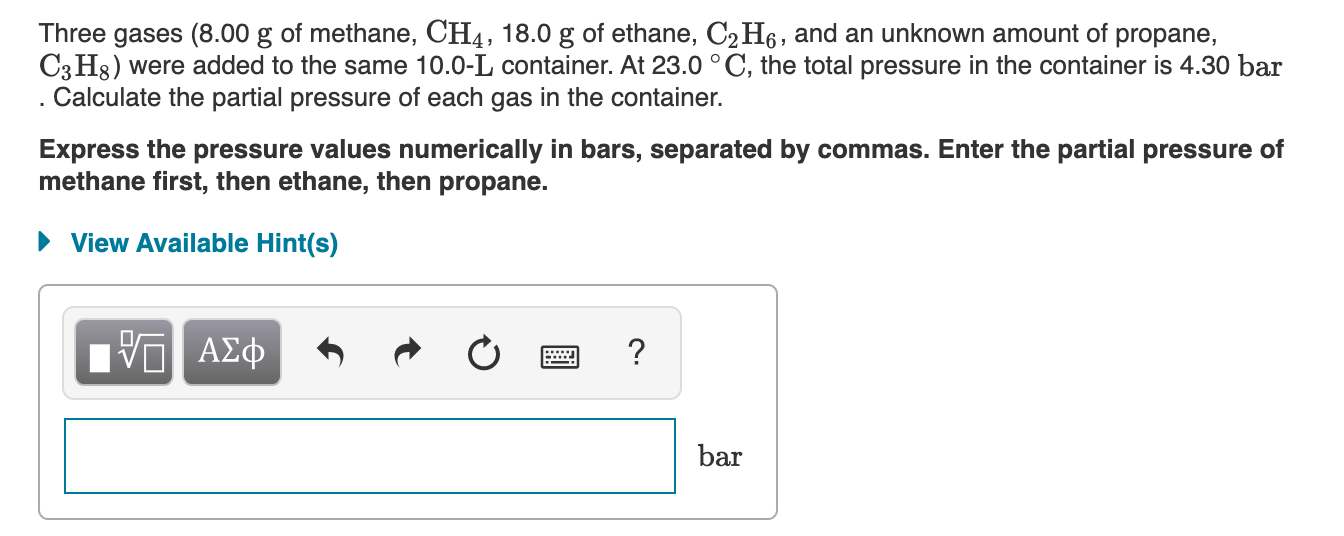
Up to 256 cash back A gaseous mixture of O2 and N2 contains 408 nitrogen by mass. Three gases 800 g of methane CH4 180 g of ethane C2H6 and an unknown amount of propane C3H8 were added to the same 100-L container. 200 ml of a gaseous mixture containing coco2n2 on complete combustion in just sufficient amount of o2 showed contraction of 40 mlwhen the resulting gases were passed through koh solution it reduces by 50 then calculate the volume ratio of co2con2 in original mixture.
What is the partial pressure of oxygen in the mixture if the total pressure is 445 mmHg. Gaseous and liquid states Solutions 2. Get the answer to this question and access a vast question bank that is tailored for students.
M is the molecular mass of gas mixture. Also remember that I have approximated some answers. A gas mixture with a total pressure of 760 mmHg contains each of the following gases at the indicated partial pressures.
What is the partial pressure of oxygen in the mixture if the total pressure is 765 mmHg shaelycole9389 is waiting for your help. P oxygen 375 mmHg. What is the partial pressure of oxygen in the mixture if the total pressure is 345 mmHg.
A mixture of gases contains 075 mol N2 030 mol O2 and 015 mol CO2. A gaseous mixture of N2 O2 containing 60 by mole N2 is allowed to react with each other according to the following equaion N2 202 - 2NO2 If 92 gm of NO2 is formed find the mole of N2 present in the mixture. A gaseous compound is 304 nitrogen and 696 oxygen by mass.
A gaseous mixture of O2 and N2 contains 368 nitrogen by mass. Experts are tested by Chegg as specialists in their subject area. Mole fraction of O2 - 19.
Remember the data I have gives RAm oxygen as 16 and nitrogen as 14 so O2 32 and N2 28. At 230 C the total. What is the partial pressure of Oxygen in the mixture if the total pressure is 285 mmHg.
A 100 L flask contains a mixture of methane and argon gasses at 25 degree C. New questions in Chemistry. The mol fraction of the O2.
A Gaseous mixture of O2 and N2 contains 358 nitrogen by mass. Add your answer and earn points. Calculate the total kinetic energy of gaseous mixture.
- Chemical Engineering Mcqs - Stoichiometry Mcqs for Chemical. A gaseous mixture of O2 and N2 contains 398 nitrogen by mass. P is the pressure and given value of it is 1 a t m.
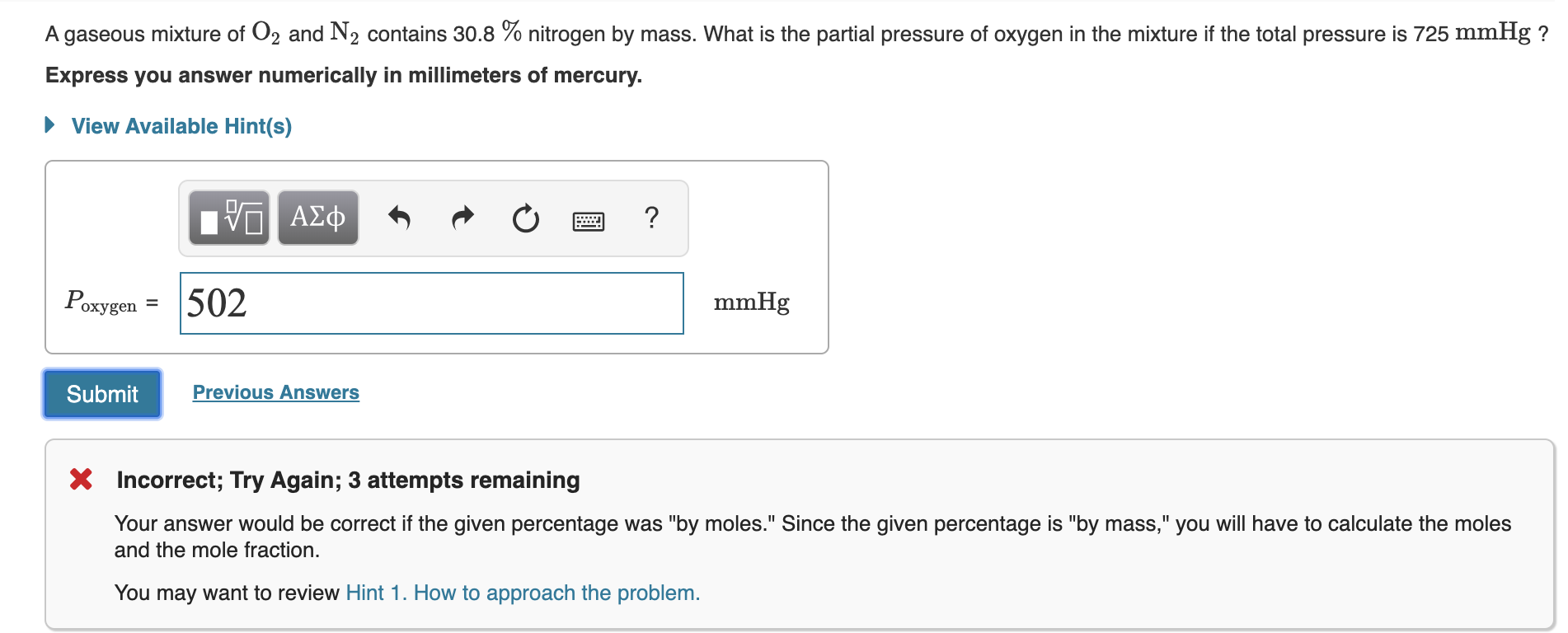
A gaseous mixture of O2 and N2 contains 318 nitrogen by mass. Who are the experts. What is the partial pressure of oxygen in the mixture if the total pressure is 725 mmHg.
A gaseous mixture of O2 and N2 contains 318 nitrogen by mass. A gaseous mixture contains 14 kg of N2 16 kg of O2 and 17 kg of NH3. CONSIDER A MIXTURE OF N2 AND O2 AT STP DENSITY IS GIVEN TO BE 13 gL CONSIDER THE TOTAL VOLUME TO BE 1L THEN THE TOTAL WEIGHT OF THE MIXTURE WILL BE 13 gm SINCE DENSITYMASSVOLUME.
A gaseous mixture of O2 and N2 contains 308 nitrogen by mass. If the total pressure of the mixture is 156 atm what is the partial pressure of each component. The mass of argon present is 228 g and the mole fraction of methane in the mixture is 0650.

Solved A Gaseous Mixture Of O2 H2 And N2 Has A Total Pressure O Chegg Com
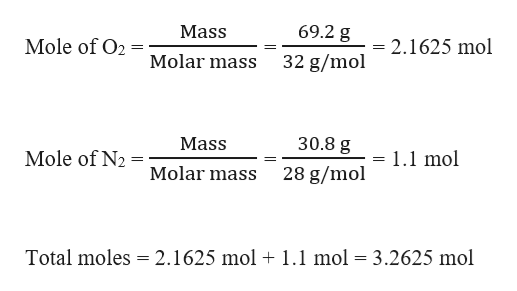
Answered A Gaseous Mixture Of O2 And N2 Contains Bartleby

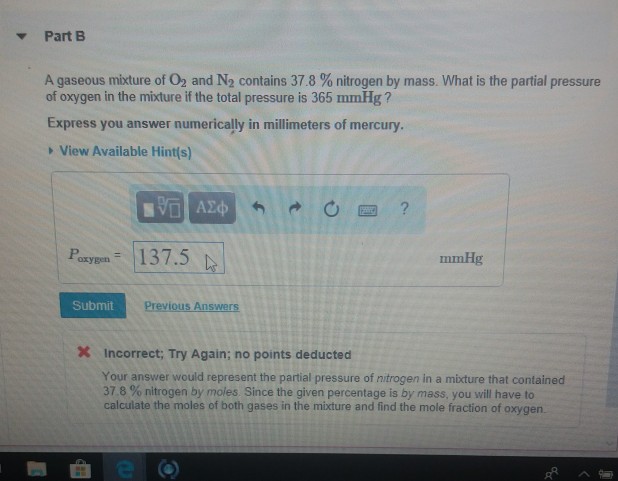
A Gaseous Mixture Of O2 And N2 Contains 37 8 Nitrogen By Mass What Is The Partial Pressure Of Brainly Com

A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 4 By Weight Therefore

Solved I Think The First Question Is Done By Using Molar Chegg Com
Solved Part B A Gaseous Mixture Of O2 And N2 Contains 37 8 Chegg Com

A Gaseous Mixture Containing 50 G Of Nitrogen And 10 G Of Oxygen Were Enclosed In A Vessel Of 10 L Capacity At 27 C Calculate A The Number Of Moles Of Each
Answered A Gaseous Mixture Of O2 And N2 Contains Bartleby

A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 8 By Mass The Ratio Of Their Youtube

A Gases Mixture Contains Oxygen And Nitrogen In The Ratio 1 4 By Weight Therefore The Rati Youtube

A Gaseous Mixture Of O2 And X Containing 20
12 00 G Of A Gaseous Mixture Of He And Methane Was Taken In A Container And To The Mixture 8 00g Of Oxygen Gas Was Added At Same Temperature Sarthaks Econnect
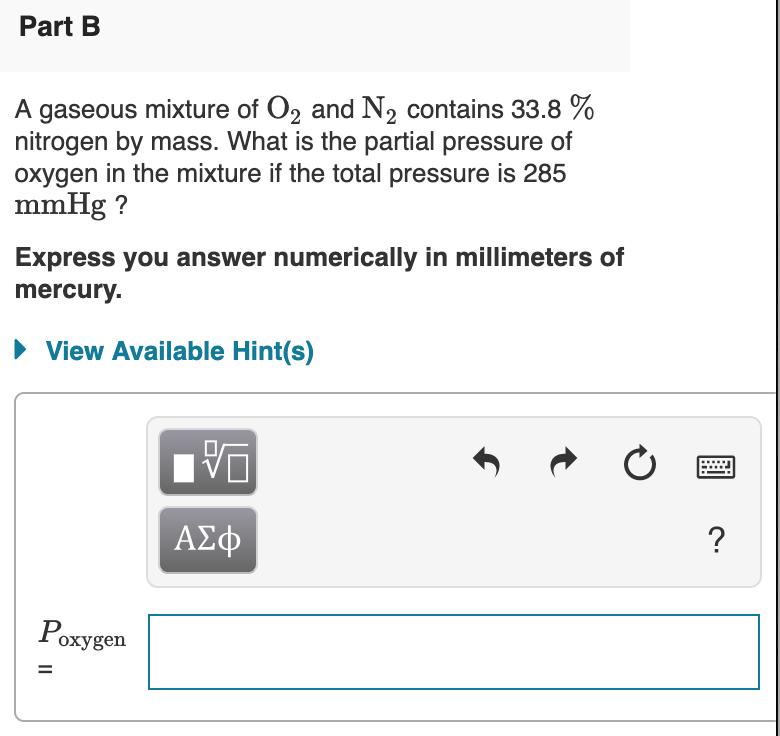
Solved Part B A Gaseous Mixture Of O2 And N2 Contains 33 8 Chegg Com

A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 4 By Weight Therefore

A Gaseous Mixture Of O2 And N2 Contains 39 8 Nitrogen By Mass What Is The Homeworklib
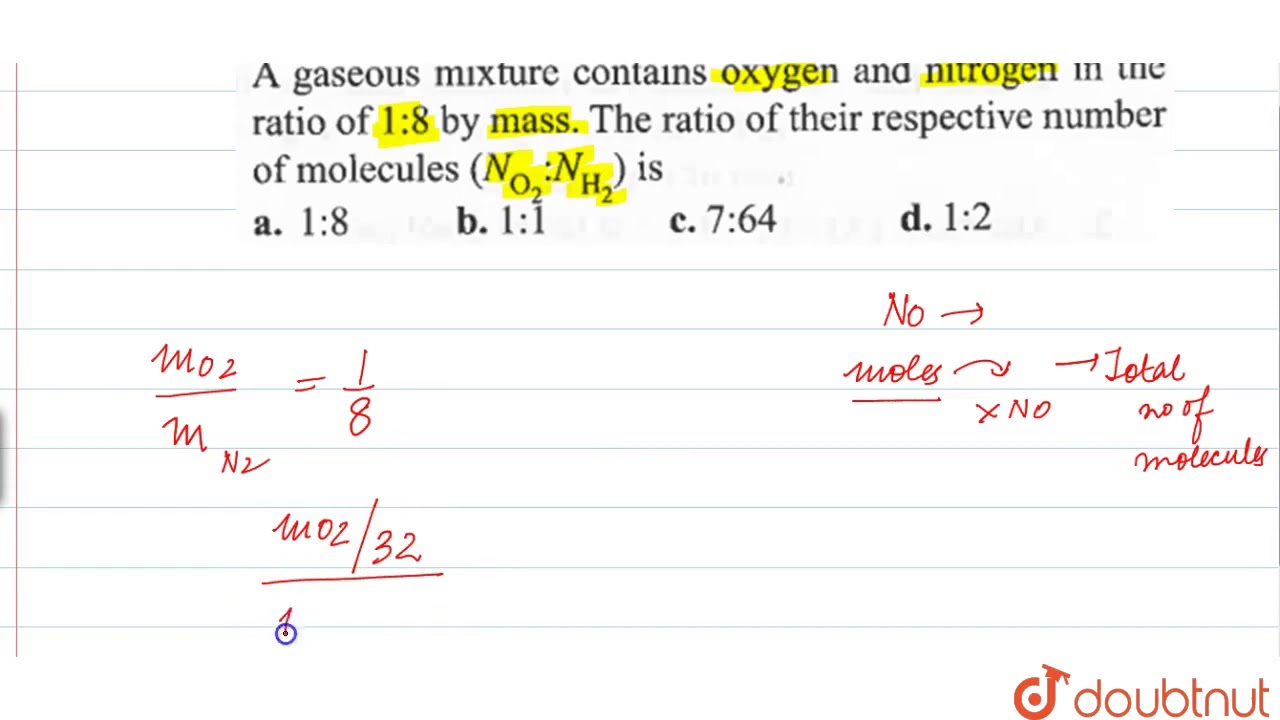
A Gaseous Mixture Contains Oxygen And Nitrogen In The Ratio Of 1 8 By Mass The Ratio Youtube
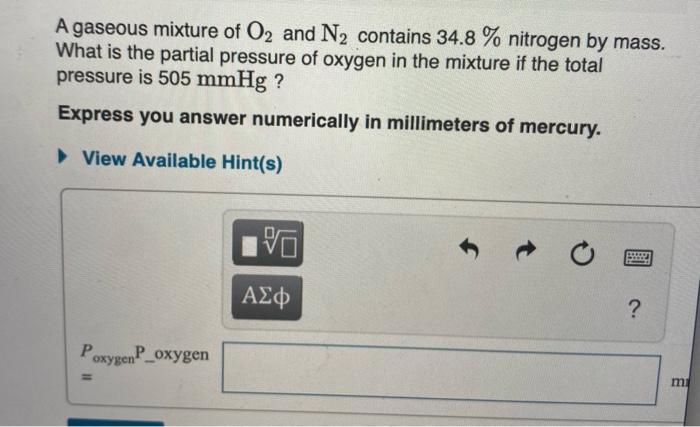
Solved A Gaseous Mixture Of O2 And N2 Contains 34 8 Chegg Com
Solved A Gaseous Mixture Of O2 And N2 Contains 35 8 Chegg Com
Solved A Gaseous Mixture Of O2 And N2 Contains 38 8 Chegg Com
Comments
Post a Comment